British American Tobacco
Organigram posts mixed results but touts international growth after Motif deal

AJNA BioSciences
FDA clears DeFloria’s cannabis-based autism treatment for phase 2 trial
autism
DeFloria advances cannabis-based autism drug through early trial
-

 California Cannabis Updates1 year ago
California Cannabis Updates1 year agoAlert: Department of Cannabis Control updates data dashboards with full data for 2023
-

 Breaking News1 year ago
Breaking News1 year agoConnecticut Appoints The US’s First Cannabis Ombudsperson – Yes there is a pun in there and I’m Sure Erin Kirk Is Going To Hear It More Than Once!
-

 best list1 year ago
best list1 year ago5 best CBD creams of 2024 by Leafly
-

 Business12 months ago
Business12 months agoEU initiative begins bid to open access to psychedelic therapies
-

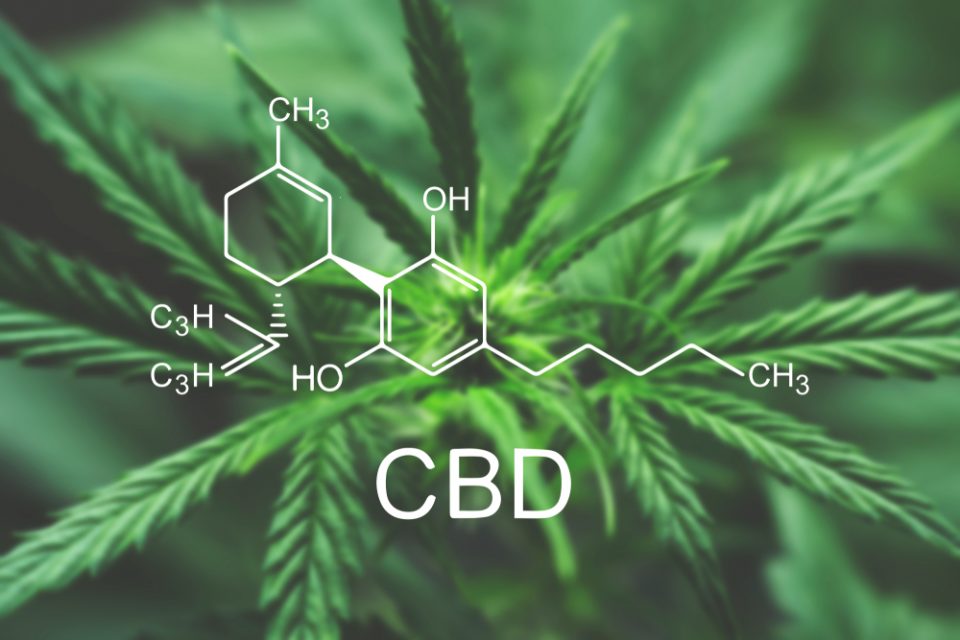
 cbd1 year ago
cbd1 year agoNew Study Analyzes the Effects of THCV, CBD on Weight Loss
-

 Bay Smokes1 year ago
Bay Smokes1 year agoFree delta-9 gummies from Bay Smokes
-

 cannabis brands12 months ago
cannabis brands12 months agoDiscover New York’s dankest cannabis brands [September 2024]
-

 autoflower seeds12 months ago
autoflower seeds12 months ago5 best autoflower seed banks of 2024 by Leafly