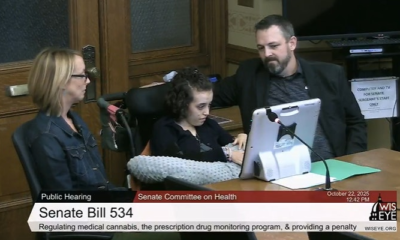
A Nebraska legislative committee voted 5-3 against advancing a bill designed to implement and regulate the state’s medical cannabis program, leaving legislators and advocates searching for alternative paths forward, according to the Nebraska Examiner.
The General Affairs Committee rejected Legislative Bill 677, sponsored by State Sen. Ben Hansen of Blair, during a Thursday vote where committee members declined to offer amendments to the legislation, the publication reported.
“I don’t want to shut all the doors right now, but some doors are closing, and they’re closing fast, and so we have to act,” Hansen told reporters after the vote, according to the Examiner.
Nebraska voters approved medical cannabis in November 2024, with residents legally permitted to possess up to 5 ounces with a healthcare practitioner’s recommendation since mid-December. However, the regulatory commission created by the ballot initiative lacks effective power and funding to regulate the industry.
Hansen described his legislation as “a must” for 2025 to prevent a “Wild West” scenario in the state’s cannabis market. The bill would have expanded regulatory structure through the Nebraska Medical Cannabis Commission and extended deadlines for regulations and licensing to allow more time for implementation, the Examiner noted.
Committee disagreements centered on proposed restrictions. A committee amendment would have prohibited smoking cannabis and the sale of flower or bud products while limiting qualified healthcare practitioners to physicians, osteopathic physicians, physician assistants or nurse practitioners who had treated patients for at least six months.
The amendment also would have limited qualifying conditions to 15 specific ailments including cancer, epilepsy, HIV/AIDS, and chronic pain lasting longer than six months.
State Sen. Bob Andersen of Sarpy County opposed allowing vaping due to concerns about youth drug use, while committee chair Rick Holdcroft suggested selling cannabis flower would be “a gateway toward recreational marijuana,” a claim Hansen “heavily disputed,” according to the Examiner.
Hansen now faces a difficult path forward, requiring at least 25 votes to pull the bill from committee and then needing 33 senators to advance it across three rounds of debate, regardless of filibuster attempts.
Crista Eggers, executive director of Nebraskans for Medical Marijuana, remained optimistic despite the setback.
“This will not be the end,” Eggers said, according to the outlet. “Giving up has never been an option. Being silenced has never been an option. It’s not over. It’s not done.”
The legislative impasse is further complicated by ongoing litigation. Former state senator John Kuehn has filed two lawsuits challenging the voter-approved provisions, with one appeal pending before the Nebraska Supreme Court. The state’s Attorney General is also trying to do something about the hemp question, akin to other states across the country.

 California Cannabis Updates1 year ago
California Cannabis Updates1 year ago
 Breaking News1 year ago
Breaking News1 year ago
 best list1 year ago
best list1 year ago
 Business1 year ago
Business1 year ago
 Business1 year ago
Business1 year ago
 cbd1 year ago
cbd1 year ago
 Bay Smokes1 year ago
Bay Smokes1 year ago
 autoflower seeds1 year ago
autoflower seeds1 year ago